Chemical equilibrium is the key to understanding how reactions behave in various situations. In essence, a reaction reaches a chemical equilibrium when the forward and reverse reactions occur at the same rate, resulting in no net change in the concentrations of reactants and products.
Whether you're a student, researcher, or simply curious about the world of chemistry, grasping the principles of chemical equilibrium is essential. By understanding how factors like temperature, pressure, and concentration affect the equilibrium state, we can optimize chemical processes and predict the behavior of chemical systems for applications in all kinds of industries.
Table of Contents:
- What Is Chemical Equilibrium?
- The Equilibrium Constant and Reaction Quotient
- Factors Influencing Chemical Equilibrium
- Applications of Chemical Equilibrium
- Calculating Equilibrium Concentrations
- Thermodynamics of Chemical Equilibrium
- Conclusion
What Is Chemical Equilibrium?
Chemical equilibrium is a state where the forward and reverse chemical reactions happen at the same rate, so the amounts of reactants and products remain constant. It's a dynamic process, with reactions still occurring on the molecular level, but no net change is observed regarding the quantity of substances in the reaction.
Definition of Chemical Equilibrium
Chemical equilibrium is reached when the forward and reverse reaction rates are equal—reactants are converted to products at the same rate that products are converted back into reactants—so that the concentrations of reactants and products stay constant over time. The system appears at rest macroscopically, but reactions continue at the molecular level.
Multiple factors affect the rate of a chemical reaction, including:
- Changes in concentration
- Changes in pressure
- Changes in temperature
- Presence of a catalyst
Le Chatelier's principle states that when an equilibrium system is disturbed, it will shift in the direction that counteracts the disturbance to re-establish equilibrium. A change in concentration, pressure, or temperature can alter the equilibrium; adding more of a particular reactant, for instance, can move the equilibrium to the right (towards the products) since there’s a higher concentration of reactants available to convert into products.
Catalysts, however, speed up the forward and reverse reaction, so the relative rates remain constant, and therefore the equilibrium does not shift to the right or left. We will cover catalysts more in a further section.
Teaching Tools
Wireless Temperature Sensor with Display (PS-4201)
The Wireless Temperature Sensor with Display is a must-have for any chemistry, biology, or environmental science course. Equally capable in the lab or field, the sensor eliminates the hassle of cables, reducing spills and improving safety.Importance of Chemical Equilibrium
Understanding chemical equilibrium is crucial for optimizing many processes:
- Respiration and Oxygen Transport: Hemoglobin binding to oxygen in the blood involves equilibrium between oxygen and deoxygenated hemoglobin.
- Acid-Base Reactions: Buffers in biological systems (e.g., bicarbonate buffer in blood) maintain pH through equilibrium between weak acids and their conjugate bases.
- Photosynthesis: The balance between CO₂, water, and glucose is maintained by chemical equilibrium during the photosynthetic process in plants.
- Ammonia Production (Haber Process): The production of ammonia from nitrogen and hydrogen is a key industrial application of chemical equilibrium.
- Water Dissociation (Self-Ionization): The equilibrium between water molecules and hydronium (H₃O⁺) and hydroxide (OH⁻) ions is critical in many chemical and biological processes.
- Electrochemical Cells: In batteries and fuel cells, the chemical reactions that generate electricity are governed by equilibrium between oxidation and reduction processes.
- Carbonate System in Oceans: The equilibrium between dissolved CO₂, carbonic acid, and bicarbonate/carbonate ions regulates ocean pH and carbon storage.
- Protein Folding: The process of protein folding into its functional structure involves equilibrium between different conformations of the protein.
- Drug Binding to Receptors: Many drugs function by binding to specific receptors in equilibrium, where the drug and receptor interaction determines the drug's effectiveness.
- Solubility of Gases in Liquids: Henry's Law describes the equilibrium between a gas dissolved in a liquid and the gas in the air above it, such as oxygen or carbon dioxide in water.
The Equilibrium Constant and Reaction Quotient
The equilibrium constant $ \left ( K \right ) $ is a mathematical expression that relates the concentrations of reactants and products at equilibrium. For a general reaction, the equilibrium constant is a numerical value that expresses the ratio of the concentrations (or partial pressures for gases) of products to reactants at equilibrium, each raised to the power of their respective stoichiometric coefficients.
For a general reaction of the form:
$ \mathit aA + bB \, \rightleftharpoons \, cC + dD $
Where:
$ \bullet\hspace{6pt}\mathit A $ and $ \mathit B $ are reactants
$ \bullet\hspace{6pt}\mathit C $ and $ \mathit D $ are products
$ \bullet\hspace{6pt}\mathit a, b, c, $ and $ \mathit d \;$ are the stoichiometric coefficients,
the equilibrium constant expression is written as:
$ \mathit {K}_c = \Large { \frac {\left [ C \right ]^c \left [ D \right ]^d} {\left [ A \right ]^a \left [ B \right ]^b} } $
Where:
$ \bullet\hspace{6pt}\mathit {K}_c $ is the equilibrium concentrations (molarity, mol/L),
$ \bullet\hspace{6pt}\left [ A \right ], \left [ B \right ], \left [ C \right ], $ and $ \left [ D \right ] $ are the equilibrium concentrations of the reactants and products.
$ \bullet\hspace{6pt} $ Brackets denote the molar concentrations at equilibrium.
For gas-phase reactions, the equilibrium constant can also be expressed in terms of partial pressures $ \left ( \mathit K_p \right ) $:
$ \mathit {K}_p = \Large { \frac {\left ( P_C \right )^c \left ( P_D \right )^d} {\left (P_A \right )^a \left ( P_B \right )^b} } $
Where:
$ \bullet\hspace{6pt}\mathit {K}_p $ is the equilibrium constant in terms of partial pressures,
$ \bullet\hspace{6pt}\mathit P_A, P_B, P_C, P_D $ are the partial pressures of the reactants and products at equilibrium.
Key Points:
$ \bullet\hspace{6pt}\mathit K > 1 $ indicates that, at equilibrium, products are favored.
$ \bullet\hspace{6pt}\mathit K < 1 $ indicates that, at equilibrium, reactants are favored.
$ \bullet\hspace{6pt} $The equilibrium constant is temperature-dependent; a change in temperature (among other factors, explained in this article) will alter its value.
Teaching Tools
Wireless pH Sensor with Display (PS-4204)
The Wireless pH Sensor with Display is a must-have for any chemistry, biology, or environmental science course. Equally capable in the lab or field, the sensor eliminates the hassle of cables, reducing spills and improving safety.Relationship Between Equilibrium Constant and Reaction Quotient
The reaction quotient $ \left ( \mathit Q \right ) $ is a mathematical expression similar to the equilibrium constant $ \left ( \mathit K \right ) $ but is used to describe the relative concentrations (or partial pressures) of reactants and products at any point during a reaction, not just at equilibrium.
For a general reaction:
$ \mathit aA + bB \, \rightleftharpoons \, cC + dD $
The reaction quotient $ \left ( \mathit Q \right ) $ is written as:
$ \mathit {Q}_c = \Large { \frac {\left [ C \right ]^c \left [ D \right ]^d} {\left [ A \right ]^a \left [ B \right ]^b} } $
Where:
$ \bullet\hspace{6pt}\mathit {Q}_c $ is the reaction quotient in terms of concentrations,
$ \bullet\hspace{6pt}\left [ A \right ], \left [ B \right ], \left [ C \right ], $ and $ \left [ D \right ] $ are the concentrations of the reactants and products at any point in time (not necessarily
at equilibrium).
Similarly, for gas-phase reactions, the reaction quotient in terms of partial pressures $ \left ( \mathit Q_p \right ) $ is:
$ \mathit {Q}_p = \Large { \frac {\left ( P_C \right )^c \left ( P_D \right )^d} {\left (P_A \right )^a \left ( P_B \right )^b} } $
Where:
$ \bullet\hspace{6pt}\mathit {Q}_p $ is the reaction quotient in terms of partial pressures,
$ \bullet\hspace{6pt}\mathit P_A, P_B, P_C, P_D $ are the partial pressures of the reactants and products.
Using the Reaction Quotient to Predict Equilibrium
When trying to forecast the direction of a reaction's progression, the reaction quotient stands out as a trusted indicator of the path to equilibrium, painting a clear picture of the potential outcome.
$ \bullet\hspace{6pt} $ If $ \mathit Q = K $: The system is at equilibrium. No net change in the concentrations of reactants and products occurs.
$ \bullet\hspace{6pt} $ If $ \mathit Q < K $: The system is not at equilibrium, and there is a higher concentration of reactants relative to products. The reaction will shift to the right (towards products) to reach equilibrium.
$ \bullet\hspace{6pt} $ If $ \mathit Q > K $: The system is not at equilibrium, and there is a higher concentration of products relative to reactants. The reaction will shift to the left (towards reactants) to reach equilibrium.
Chemists can manipulate the concentrations of reactants or products to drive a reaction in the desired direction, as the system will always strive to reach equilibrium. The reaction quotient is useful for predicting the direction that a reaction will proceed to achieve equilibrium. While $K$ is fixed at a given temperature, $Q$ can change as the reaction progresses until it reaches $K$ when equilibrium is established. By comparing the values of $Q$ and $K$, we gain insight into the current state of the reaction mixture relative to the equilibrium position.
Teaching Tools
Essential Chemistry Solution
This rigorous yet accessible textbook includes core chemistry topics that cover a complete year of instruction. The lessons follow the 5E model and include tools for ELL students, as well as tools for students with different learning styles. And the curriculum aligns to your standards for both regular and advanced coursework.
Factors Influencing Chemical Equilibrium
Chemical equilibrium is influenced by changes in concentration, temperature, pressure, and the presence of catalysts, as described by Le Châtelier's Principle. When the concentration of reactants or products changes, the system adjusts to minimize the disturbance by shifting the reaction toward the side that compensates for the change. Temperature changes affect the equilibrium constant by favoring either the exothermic or endothermic direction, depending on whether heat is added or removed. Pressure primarily affects reactions involving gases, where an increase in pressure will favor the side with fewer gas molecules, while catalysts speed up the attainment of equilibrium but do not alter the position of the equilibrium itself.
Le Chatelier's Principle
Le Chatelier's principle states that when a system in balance is disturbed, it seeks to counterbalance the disruption by shifting its state. Chemists rely on this principle to predict how external factors will affect the equilibrium.
Effect of Concentration Changes
Changing the concentration of a reactant or product will shift the equilibrium position:
Increasing the concentration of a reactant will cause the equilibrium to shift towards the products, while increasing the concentration of a product will shift the equilibrium towards the reactants. This is in accordance with Le Chatelier's principle, as the system adjusts to counteract the concentration change.
Impact of Pressure Changes
Pressure changes affect gaseous equilibria:
Increasing the pressure will shift the equilibrium towards the side with fewer moles of gas, as this minimizes the pressure increase. Conversely, decreasing the pressure will shift the equilibrium towards the side with more moles of gas. Reactions involving only solids and liquids are not affected by pressure changes.
Temperature Effects on Equilibrium
Temperature changes affect the equilibrium depending on whether the reaction is exothermic or endothermic:
For exothermic reactions, increasing the temperature decreases $K$, as the reaction shifts toward the reactants to absorb excess heat. Conversely, for endothermic reactions, raising the temperature increases $K$, as the system shifts toward the products, favoring heat absorption to restore equilibrium.
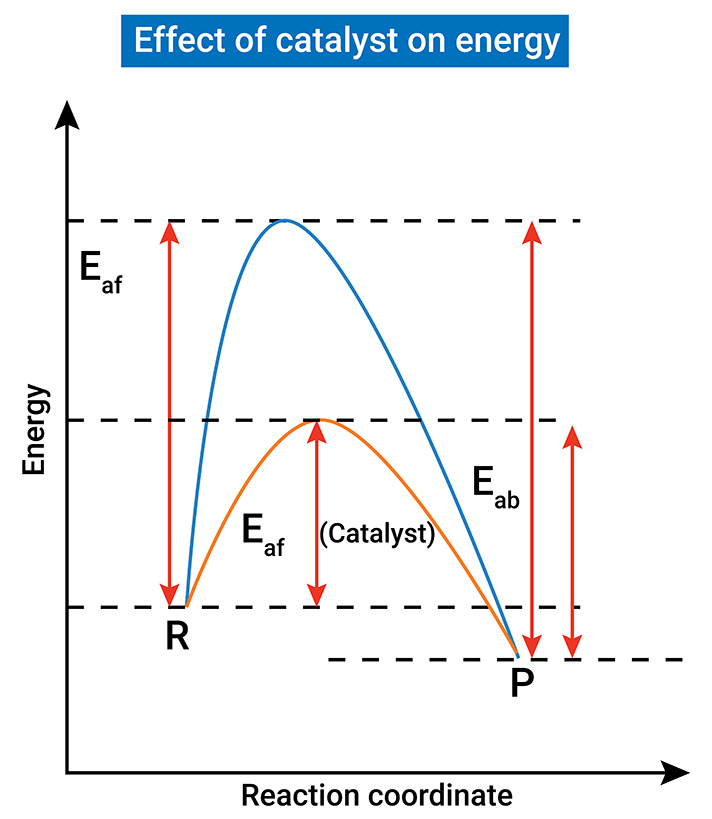
Role of Catalysts
Catalysts act as a helping hand in biochemical reactions. By nature, catalysts accelerate both forward and reverse reactions by an equal amount, in turn not changing the relative energies of the reactants and products. For this reason, they do not affect the equilibrium position or the value of the equilibrium constant; in other words, catalysts do not affect the equilibrium constant $ \left ( K \right ) $ because they speed up both the forward and reverse reactions equally, allowing the system to reach equilibrium faster without changing the equilibrium position.
Applications of Chemical Equilibrium
From industrial processes to environmental and biological systems, chemical equilibrium principles are harnessed to make them work effectively.
Solubility Equilibria
Solubility equilibria involve the dissolution of ionic compounds in aqueous solutions:
The solubility product constant $ \left ( K_{sp} \right ) $ is a measure of the delicate balance between a solid salt and its dissolved ions in a liquid. It's what makes precipitation reactions possible, stalactites and stalagmites grow, and water purification processes effective. The solubility product constant indicates the extent to which a salt dissolves in water, with a larger $ K_{sp} $ meaning greater solubility and a smaller $ K_{sp} $ meaning lower solubility.
Acid-Base Equilibria
Acid-base equilibria involve the reversible reactions between acids and bases in which protons $ \left ( \mathsf {H^+} \right ) $ are transferred, and the position of the equilibrium depends on the relative strengths of the acid and base involved. The equilibrium constant for these reactions, known as $ \left ( \mathsf {K_a} \right ) $ for acids and $ \left ( \mathsf {K_b} \right ) $ for bases, quantifies the extent of dissociation, with stronger acids and bases having larger values of $ \left ( \mathsf {K_a} \right ) $ or $ \left ( \mathsf {K_b} \right ) $.

Gas Phase Equilibria
Gas phase equilibria involve reactions between gaseous species. Here are some examples:
$ \bullet\hspace{6pt} \mathsf {P_4(s) + 6\,Cl_2(g) ⇌ 4\,PCl_3(l)} $
$ \bullet\hspace{6pt} \mathsf {PCl_3(g) + Cl_2(g) ⇌ PCl_5(g)} $
$ \bullet\hspace{6pt} \mathsf {PCl_3(g) + 3\,NH_3(g) ⇌ P(NH_2)_3(g) + 3\,HCl(g)} $
To reach a balance in a chemical reaction happening in a gas mixture, the equilibrium constant for gas phase reactions is used, which is often described by the equilibrium constant $ \left ( \mathsf {K_p} \right ) $ in terms of partial pressures, as it tells us the partial pressures of reactants and products. Applications of this concept include the synthesis of ammonia using the Haber-Bosch process and the formation of nitrogen oxides in car engines.
Equilibrium in Combustion and Detonation
In combustion, chemical equilibrium is reached when the rate of fuel oxidation equals the rate of product formation, typically involving gases like oxygen and carbon dioxide, though complete equilibrium is rarely achieved in practical systems due to ongoing reactions. Detonation, a form of combustion that involves a shock wave, operates far from equilibrium as the chemical reactions occur almost instantaneously, leaving little time for equilibrium to establish. However, post-detonation gases may eventually reach chemical equilibrium as they cool and react more slowly, stabilizing the final product composition.
The duo of Sanford Gordon and Bonnie J. McBride changed the game with the creation of NASA's Chemical Equilibrium with Applications, a comprehensive computer program capable of calculating the intricate compositions and thermodynamic properties of complex mixtures like those found in rocket combustion chambers and detonation waves.
Calculating Equilibrium Concentrations
To better understand the balance of reactants and products at equilibrium, we must set up mathematical equations and solve for the unknown values that indicate the concentrations.
Setting Up Equilibrium Equations
To calculate equilibrium concentrations, one must first set up equilibrium equations based on the balanced chemical equation and the initial concentrations of reactants and products. The equilibrium constant expression is then written in terms of the unknown equilibrium concentrations.
Solving for Equilibrium Concentrations
Solving for equilibrium concentrations involves applying the equilibrium constant expression for a given reaction to determine the concentrations of reactants and products at equilibrium. First, an ICE (Initial, Change, Equilibrium) table is typically constructed to track the initial concentrations, the changes that occur as the system approaches equilibrium, and the final equilibrium concentrations. The equilibrium constant $ \left ( \mathsf {K_c} \right ) $ or $ \left ( \mathsf {K_p} \right ) $ is then used to set up an equation relating the concentrations of reactants and products, based on the stoichiometry of the reaction. Solving the equation, often a quadratic or simpler algebraic equation, gives the equilibrium concentrations. Depending on the system, approximations like assuming small changes can simplify calculations, especially when the equilibrium constant is very small or very large.
Teaching Tools
Wireless Conductivity Sensor with Display
The Wireless Conductivity Sensor with Display is a convenient way to manually measure both conductivity and total dissolved solids in solution in real-time using the bright OLED display for indoor or outdoor labs; or, transfer data wirelessly to a device to graph and analyze data using PASCO software.Using Equilibrium Constants
Equilibrium constants are highly useful in predicting the extent of a chemical reaction and understanding the behavior of a system at equilibrium. They allow chemists to quantify the relative concentrations of reactants and products, providing insight into whether a reaction favors the formation of products or reactants. With known equilibrium constants, one can calculate equilibrium concentrations, determine the direction of reaction shifts, and assess how changes in conditions such as concentration, temperature, or pressure will affect the system.
Equilibrium constants are also crucial in industrial applications, such as optimizing reactions for product yield, and in natural processes like biological buffer systems and environmental chemistry. Overall, they serve as a key tool for controlling and predicting the outcome of chemical processes.

Simplifying Assumptions
Bypassing some details can actually make it easier to understand the complexity of molecular interactions, as we aim to arrive at the essential concentrations of equilibrium states.
If the equilibrium constant is very large or very small, the equilibrium concentrations of some species in the reaction may be negligible. Additionally, if the initial concentration of a reactant is much larger than the others, it may be assumed to remain constant throughout the reaction.
Thermodynamics of Chemical Equilibrium
Chemical equilibrium is a delicate balance that's heavily influenced by a system's thermodynamic properties. A change in the reaction conditions can significantly impact the value of the equilibrium constant, reflecting the interplay between the reactants and products.
Gibbs Free Energy and Equilibrium
The Gibbs free energy $ \left ( \mathsf {G} \right ) $ is a thermodynamic quantity that determines the spontaneity of a reaction:
At equilibrium, the change in Gibbs free energy $ \left ( \Delta\mathsf {G} \right ) $ is equal to zero. The standard Gibbs free energy change $ \left ( \Delta\mathsf {G}^\circ \right ) $ is related to the equilibrium constant by the equation $ \Delta\mathsf {G}^\circ = \mathsf {-RT\;ln(K)} $, where $ \mathsf {R} $ is the gas constant and $ \mathsf {T} $ is the absolute temperature.
Entropy and Enthalpy Changes
The entropy change $ \left ( \Delta\mathsf {S} \right ) $ and enthalpy change $ \left ( \Delta\mathsf {H} \right ) $ of a reaction contribute to the Gibbs free energy change and thus affect the equilibrium position:
Reactions with a positive entropy change and a negative enthalpy change are more likely to have a larger equilibrium constant and proceed further towards completion.
Equilibrium and Thermodynamic Stability
Equilibrium is all about stability, where a system aligns itself to minimize its free energy. By doing so, it achieves a steady state, or thermodynamic balance.
Deviations from equilibrium will result in a spontaneous process that drives the system back towards the equilibrium state. The further a system is from equilibrium, the greater the driving force for it to return to equilibrium.
Chemical equilibrium occurs when forward and reverse reactions happen at the same rate; Le Chatelier's principle states that when there is a change introduced to a system at equilibrium, the equilibrium position shifts to drive the reaction in a particular direction to counteract the effect of the change.
Conclusion
Chemical equilibrium is a cornerstone of chemistry that helps us understand the behavior of chemical reactions. It's a state of balance where the forward and reverse reactions proceed at equal rates, resulting in no net change in the concentrations of reactants and products. The equilibrium constant defines the ratio of the equilibrium concentrations of products to reactants (with each concentration raised to the power of its stoichiometric coefficient), thereby determining the position of equilibrium in a chemical reaction. This balance is crucial, as it helps us comprehend the intricate relationships between chemical reactions and their equilibrium states.
Understanding chemical equilibrium is essential for predicting reaction behavior, optimizing processes, and controlling chemical systems in both industrial and natural environments. But the importance of chemical equilibrium goes beyond just chemistry; it teaches us valuable lessons about balance and stability, and how systems react to offset stressors.